
MQA Q1 2014 - 2015
C A R D M E A S U R E S
Data source:
MQA Customer Oriented Medical Practitioner Administration System (COMPAS) DataMart
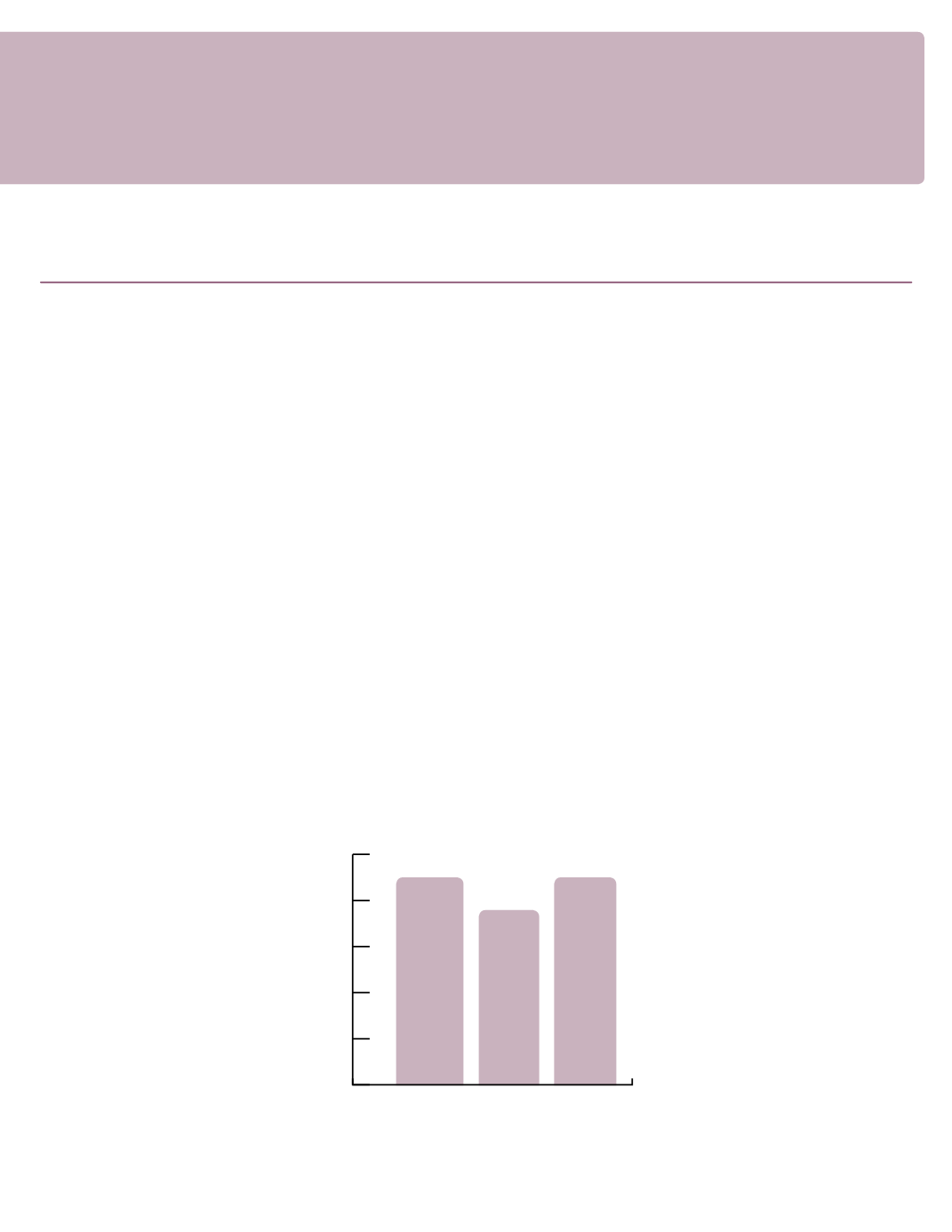
Sterile Compunding Pharmacy Inspections
FY 13-14 Q3
75.68%
FY 13-14 Q4
89.88%
FY 14-15 Q1
89.83%
0
20
40
60
80
100
This measure is calculated using the inspection end date and the inspection deficiency data. The number of
sterile compounding pharmacy inspections completed without serious deficiencies is divided by the number of
sterile compounding pharmacy inspections completed during the specified timeframe. It is important to make
sure sterile compounding pharmacies are in compliance with Florida Statutes and administrative rules and do
not pose a threat to the health, safety and welfare of the public.
Because standards for sterile compounding pharmacies are being raised by enactment of U.S. Pharmacopeia
Rule 797 taking effect Oct. 1, the Bureau of Enforcement adopted the standards into Rule 64B16-27-797 and
created new inspection forms. Monthly conference calls will be conducted with MQA inspectors to monitor how
the new standards are affecting pharmacy passage rates. All new senior pharmacists will undergo “boot camp”
training, and the training will be incorporated into annual requirements for all senior pharmacists. Inspections
will be used as an educational opportunity on the new standards, and any deficiencies will be followed up with
additional inspections. The Bureau, in conjunction with the Board of Pharmacy, will hold a public workshop in
October to inform stakeholders of the new standards.
INITIATIVE:
TARGET: 95%
MEASURE:
Percent of sterile compounding pharmacy inspections with no serious deficiencies.
DEFINITION:
14

















