
MQA Q3 2014 - 2015
BALANCED SCORECARD MEASURES
Data source:
MQA Licensing & Enforcement Information Database System (LEIDS)
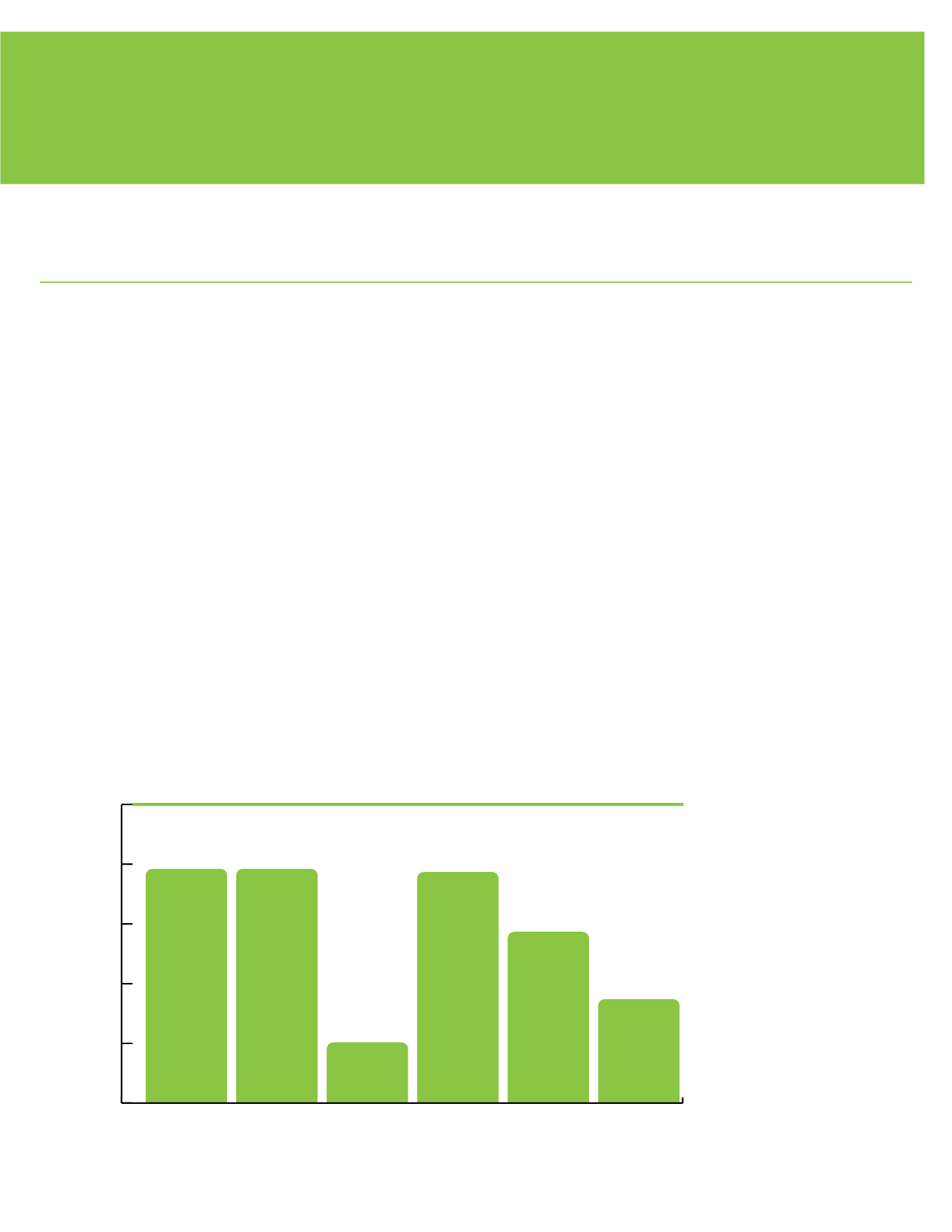
This measure is calculated using the inspection end date and the inspection deficiency data. The number of
sterile compounding pharmacy inspections completed without serious deficiencies is divided by the number of
sterile compounding pharmacy inspections completed during the specified timeframe. It is important to make
sure sterile compounding pharmacies are in compliance with Florida Statutes and administrative rules and do
not pose a threat to the health, safety and welfare of the public.
Due to the implementation of USP 797, standards for sterile compounding pharmacies were raised. The Board
of Pharmacy adopted the standards into Florida Administrative Code Rule 64B16-27.797, and the Bureau of
Enforcement adopted the standards into their inspection forms. To ensure compounded sterile drugs entering
and leaving the state are safe and adhere to USP 797 requirements, the following action steps will be carried
out. Monthly conference calls will be conducted with MQA inspectors to monitor how new standards are
affecting pharmacy passage rates, and all new senior pharmacists will undergo “boot camp” training. Annual
“boot camp” refresher training will also be mandatory for all senior pharmacists. Inspections will also be used
as an educational opportunity on the new standards, and any deficiencies will be followed up with additional
inspections.
INITIATIVE:
TARGET: 95%
MEASURE:
Percent of sterile compounding pharmacy inspections with no serious deficiencies.
DEFINITION:
16
Sterile Compunding Pharmacy Inspections
89.88
89.92
75.68
89.83
83.54
78.53
% without serious deficiencies
95
90
85
80
75
70
FY 13-14 Q3
FY 13-14 Q2
FY 13-14 Q4 FY 14-15 Q1
FY 14-15 Q2 FY 14-15 Q3
TARGET = 95%
l

















